Project Description
Publications
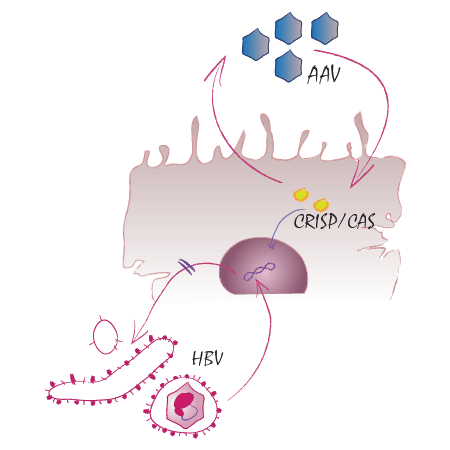
TP18: Combinatorial knock-down/knock-out strategies to reconstitute antiviral im-munity and eliminate persisting hepatitis B virus cccDNA
We aim to overcome the immune tolerance against HBV by controlling HBV antigen expression. This will be achieved by inhibiting HBV on the RNA and DNA level via RNAi and CRISPR, respectively. We expect to find that direct targeting of the HBV cccDNA and suppression of the viral transcripts, and hence of viral antigen expression, will restore HBV immunity either on its own or at least after therapeutic vaccination, and that restored B- and T-cell immunity will ultimately clear HBV in vivo. Specific aims include AAV vector fine-tuning in increasingly complex HBV infection models, targeting of checkpoints by RNAi, characterization of potential adverse effects and improving CRISPR safety.Su J, Brunner L, Ates Oz E, Sacherl J, Frank G, Kerth HA, Thiele F, Wiegand M, Mogler C, Aguilar JC, Knolle PA, Collin N, Kosinska AD, Protzer U. (2023) Activation of CD4 T cells during prime immunization determines the success of a therapeutic hepatitis B vaccine in HBV-carrier mouse models. J Hepatol. 78(4):717-730. doi: 10.1016/j.jhep.2022.12.013. Sacherl J, Kosinska AD, Kemter K, Kächele M, Laumen SC, Kerth HA, Öz EA, Wolff LS, Su J, Essbauer S, Sutter G, Scholz M, Singethan K, Altrichter J, Protzer U. (2022) Efficient stabilization of therapeutic hepatitis B vaccine components by amino-acid formulation maintains its potential to break immune tolerance. JHEP Rep. 13;5(2):100603. doi: 10.1016/j.jhepr.2022.100603.Tu T, Zehnder B, Wettengel JM, Zhang H, Coulter S, Ho V, Douglas MW, Protzer U, George J, Urban S. (2022) Mitosis of hepatitis B virus-infected cells in vitro results in uninfected daughter cells. JHEP Rep. 15;4(9):100514. doi: 10.1016/j.jhepr.2022.100514.Kim ES, Zhou J, Zhang H, Marchetti A, van de Klundert M, Cai D, Yu X, Mitra B, Liu Y, Wang M, Protzer U, Guo H. (2022) Hepatitis B virus X protein counteracts high mobility group box 1 protein-mediated epigenetic silencing of covalently closed circular DNA. PLoS Pathog. 9;18(6):e1010576. doi: 10.1371/journal.ppat.1010576. Zhang C, Freistaedter A, Schmelas C, Gunkel M, Dao Thi VL, Grimm D. (2022) An RNA Interference/Adeno-Associated Virus Vector-Based Combinatorial Gene Therapy Approach Against Hepatitis E Virus. Hepatol Commun. 6(4):878-888. doi: 10.1002/hep4.1842.Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, Feuerherd M, Cheng CC, Yazici S, Christa C, Jeske S, Lupoli G, Vogt T, Albanese M, Mejías-Pérez E, Bauernfried S, Graf N, Mijocevic H, Vu M, Tinnefeld K, Wettengel J, Hoffmann D, Muenchhoff M, Daechert C, Mairhofer H, Krebs S, Fingerle V, Graf A, Steininger P, Blum H, Hornung V, Liebl B, Überla K, Prelog M, Knolle P, Keppler OT, Protzer U. (2022) Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 28(3):496-503. doi: 10.1038/s41591-022-01715-4.Koerber N, Priller A , Yazici S, Bauer T, Cheng CC, Mijočević H, Wintersteller H, Jeske S, Vogel E, Feuerherd M, Tinnefeld K, Winter C, Ruland J, Gerhard M, Haller B, Christa C, Zelger O, Roggendorf H, Halle M, Erber J, Lingor P, Keppler O, Zehn D, Protzer U, Knolle PA. (2022) Dynamics of spike-and nucleocapsid specific immunity during long-term follow-up and vaccination of SARS-CoV-2 convalescents. Nat Commun 13, 153. https://doi.org/10.1038/s41467-021-27649-y.Bunse T, Kosinska AD, Michler T, Protzer U. (2022) PD-L1 silencing in liver using siRNAs enhances efficacy of therapeutic vaccination for chronic Hepatitis B. Biomolecules. 18;12(3):470. doi: 10.3390/biom12030470.Maurer L, El Andari J, Rapti K, Spreyer L, Steinmann E, Grimm D, Dao Thi VL. (2022) Induction of Hepatitis E virus anti-ORF3 antibodies from systemic administration of a muscle-specific adeno-associated virus (AAV) vector. Viruses. 27;14(2):266. doi: 10.3390/v14020266.Wagner KI, Mateyka LM, Jarosch S, Grass V, Weber S, Schober K, Hammel M, Burrell T, Kalali B, Poppert H, Beyer H, Schambeck S, Holdenrieder S, Strötges-Achatz A, Haselmann V, Neumaier M, Erber J, Priller A, Yazici S, Roggendorf H, Odendahl M, Tonn T, Dick A, Witter K, Mijočević H, Protzer U, Knolle PA, Pichlmair A, Crowell CS, Gerhard M, D'Ippolito E, Busch DH. (2022) Recruitment of highly cytotoxic CD8+ T cell receptors in mild SARS-CoV-2 infection. Cell Rep. 11;38(2):110214. doi: 10.1016/j.celrep.2021.110214.Schreiber S, Honz M, Mamozai W, Kurktschiev P, Schiemann M, Witter K, Moore E, Zielinski C, Sette A, Protzer U, Wisskirchen K. (2021) Characterization of a library of 20 HBV-specific MHC class II-restricted T cell receptors. Mol Ther Methods Clin Dev. 29;23:476-489. doi: 10.1016/j.omtm.2021.10.012.Manske K, Schneider A, Ko C, Knolle PA, Steiger K, Protzer U, Wohlleber D. (2021) In vivo bioluminescence imaging of HBV replicating hepatocytes allows for the monitoring of anti-viral immunity viruses. 13(11): 2273. doi: 10.3390/v13112273. Rapti K, Grimm D. (2021) Adeno-associated viruses (AAV) and host immunity – a race between the hare and the hedgehog. Front Immunol. 12: 753467. doi: 10.3389/fimmu.2021.753467.Kremer LPM, Cerrizuela S, Dehler S, Stiehl T, Weinmann J, Abendroth H, Kleber S, Laure A, El Andari J, Anders S, Marciniak-Czochra A, Grimm D, Martin-Villalba A. (2021) High throughput screening of novel AAV capsids identifies variants for transduction of adult NSCs within the subventricular zone. Mol Ther Methods Clin Dev. 16;23:33-50. doi: 10.1016/j.omtm.2021.07.001.Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, Haas DA, Huang Y, Oubraham L, Wang A, Hamad MS, Piras A, Hansen FM, Tanzer MC, Paron I, Zinzula L, Engleitner T, Reinecke M, Lavacca TM, Ehmann R, Wölfel R, Jores J, Kuster B, Protzer U, Rad R, Ziebuhr J, Thiel V, Scaturro P, Mann M, Pichlmair A. (2021) Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 594(7862):246-252. doi: 10.1038/s41586-021-03493-4. Zhang C, Freistaedter A, Schmelas C, Gunkel M, Dao Thi VL, Grimm D. (2021) An RNA interference/adeno-associated virus vector-based combinatorial gene therapy approach against Hepatitis E virus. Hepatol Commun. 6(4):878-888. doi: 10.1002/hep4.1842.Lampl S, Janas MK, Donakonda S, Brugger M, Lohr K, Schneider A, Manske K, Sperl LE, Kläger S, Küster B, Wettmarshausen J, Müller C, Laschinger M, Hartmann D, Hüser N, Perocchi F, Schmitt-Kopplin P, Hagn F, Zender L, Hornung V, Borner C, Pichlmair A, Kashkar H, Klingenspor M, Prinz M, Schreiner S, Conrad M, Jost PJ, Zischka H, Steiger K, Krönke M, Zehn D, Protzer U, Heikenwälder M, Knolle PA, Wohlleber D. Reduced mitochondrial resilience enables non-canonical induction of apoptosis after TNF receptor signaling in virus-infected hepatocytes. J Hepatol. 2020 Jun 26:S0168-8278(20)30398-6. doi: 10.1016/j.jhep.2020.06.026. (TP05, TP06, TP10, TP11, TP13, TP16, TP18) Börner K, Kienle E, Huang LY, Weinmann J, Sacher A, Bayer P, Stüllein C, Fakhiri J, Zimmermann L, Westhaus A, Beneke J, Beil N, Wiedtke E, Schmelas C, Miltner D, Rau A, Erfle H, Kräusslich HG, Müller M, Agbandje-McKenna M, Grimm D.Pre-arrayed Pan-AAV Peptide Display Libraries for Rapid Single-Round Screening. Mol Ther. 2020 Feb 13. pii: S1525-0016(20)30095-2. doi: 10.1016/j.ymthe.2020.02.009. (TP18)Michler T, Kosinska AD, Festag J, Bunse T, Su J, Ringelhan M, Imhof H, Grimm D, Steiger K, Mogler C, Heikenwalder M, Michel ML, Guzman CA, Milstein S, Sepp-Lorenzino L, Knolle P, Protzer U. 2020. Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-titer HBV Carrier Mice. Gastroenterology. 158(6):1762-1775. (TP05, TP06, TP18)Wisskirchen K*, Kah J*, Malo A, Asen T, Volz T, Allweiss L, Wettengel JM, Lütgehetmann M, Urban S, Bauer T, Dandri M*, Protzer U*. 2019. T-Cell Receptor Grafting allows Virological Control of Hepatitis B Virus Infection. J Clin Invest. 129(7):2932-2945.Hoffmann M, Aschenbrenner S, Grosse S, Rapti K, Domenger C, Fakhiri J, Mastel M, Börner K, Eils R, Grimm D, Niopek D. 2019. Cell-specific CRISPR/Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 47(13):e75.Kosinska AD, Moeed A, Kallin N, Festag J, Su J, Steiger K, Michel ML, Protzer U, Knolle PA. 2019. Synergy of therapeutic heterologous prime-boost hepatitis B vaccination with CpG-application to im-prove immune control of persistent HBV infection. Sci Rep. 9(1):10808.Bubeck F, Hoffmann M, Harteveld Z, Aschenbrenner S, Bietz A, Waldhauer M, Börner K, Fakhiri J, Schmelas C, Dietz L, Grimm D, Correia B, Eils R, Niopek D. 2018. Engineered anti-CRISPR proteins for optogenetic control of CRISPR/Cas9. Nat. Methods 15(11):924-927.Schmelas C, Grimm D. 2018. Split Cas9, not hairs – advancing the therapeutic index of CRISPR tech-nology. Biotechnol. J. 13:e1700432.Velkov S, Ott J, Protzer U, Michler T. 2018. The global Hepatitis B Virus genotype distribution estimat-ed from available genotyping data. Genes 9(10):pii: E495.Schiwon M, Ehrke-Schulz E, Oswald A, Bergmann T, Michler T, Protzer U, Ehrhardt A. 2018. One-Vector System for Multiplexed CRISPR/Cas9 against Hepatitis B Virus cccDNA Utilizing High-Capacity Adenoviral Vectors. Mol. Ther. Nucleic Acids 12:242-253.Burwitz B*, Wettengel J*, Mück-Häusl M*, Ringelhan M, Ko C, Festag MM, Hammond KB, Northrup M, Bimber BN, Jacob T, Reed JS, Reed N, Park B, Moller-Tank S, Esser K, Greene JM, Wu HL, Ab-dulhaqq S, Webb G, Sutton WF, Klug A, Swanson T, Legasse AW, Vu TQ, Asokan A, Haigwood NL, Protzer U*, Sacha J*. 2017. Hepatocytic Expression of Human Sodium-Taurocholate Cotransporting Polypeptide Enables Hepatitis B Virus Infection of Macaques. Nat. Commun. 8(1):2146.Lempp FA, Wiedtke E, Qu B, Roques P, Chemin I, Vondran FWR, Grand RL, Grimm D, Urban S. 2017. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology 66(3):703-716.Michler T, Grosse S, Mockenhaupt S, Röder N, Stückler F, Knapp B, Ko C, Heikenwälder M, Protzer U, Grimm D. 2016. Blocking sense strand activity improves potency, safety and specificity of anti-hepatitis B virus short hairpin RNA. EMBO Mol. Med. 8(9):1082-1098.