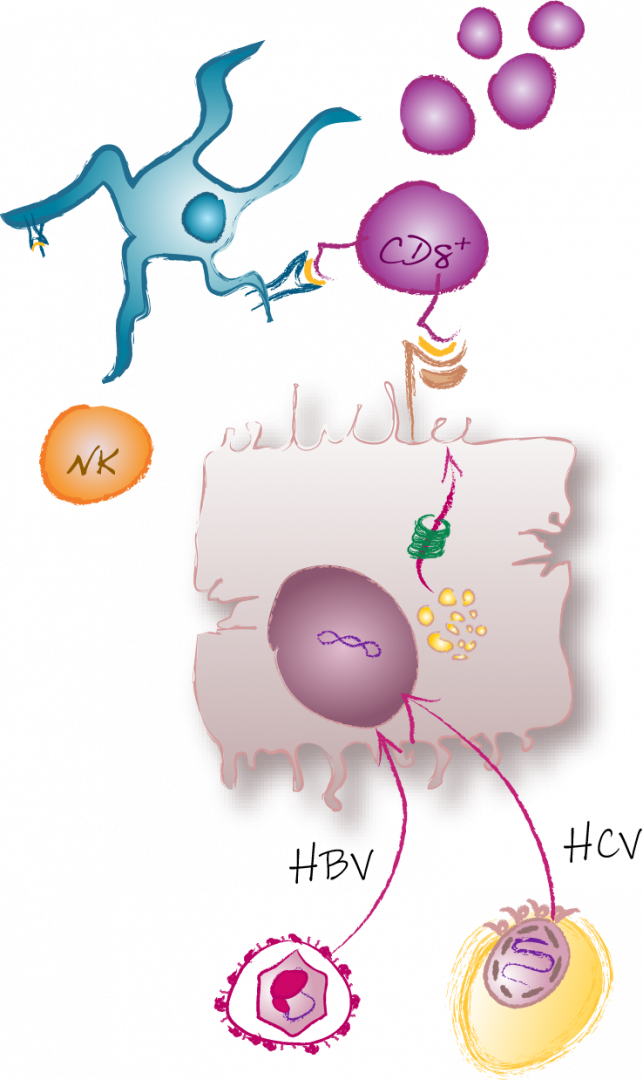
CD8+ T cells are a well-recognized cornerstone of the outcome and pathogenesis of viral hepatitis. Indeed, virus-specific CD8+ T cell responses in acute-resolving and chronic viral hepatitis diverge already early during infection resulting in an effector/memory versus an exhausted T cell response, respectively. Together with Thimme, Neumann-Haefelin and Bengsch, we demonstrated that the transcriptional regulator thymocyte selection-associated high mobility group box protein (TOX) is a relevant rheostat in this divergence of virus-specific CD8+ T cell effector/memory versus exhaustion in viral hepatitis and beyond. Still, in both, acute-resolving and chronic viral hepatitis, the virus-specific T cell response is guided by heterogeneous subsets consisting of cells with stem-like versus effector and terminally differentiated characteristics. In particular, in the last two funding periods, together with Thimme, Neumann-Haefelin, Bartenschlager and Z03 we showed that also in chronic infections with hepatitis B virus (HBV), hepatitis D virus (HDV), hepatitis C virus (HCV) and hepatitis E virus (HEV) distinct subsets of memory-like and terminally exhausted cells emerge. These memory-like subsets are characterized by the expression of the IL-7 receptor alpha-chain CD127 and the transcription factor TCF-1, exhibit a superior proliferative capacity, maintain the pool of virus-specific CD8+ T cells during chronic infection, are in a progenitor-progeny relationship to terminally exhausted cells, survive independently of antigen and provide recall responses after cessation of chronic antigen stimulation. Hence, memory-like subsets comprise stem-like features with the potential to maintain virus-specific CD8+ T cell immunity and the capacity to give rise to a heterogeneous T cell response in the context of chronic viral hepatitis. However, the identification of the stem-like/progenitor cells within the memory-like subsets and the comparison of the stem-like characteristics and determinants in acute-resolving versus chronic infections with different hepatitis viruses remain open issues.
Based on the hypothesis that stem-like cells drive virus-specific CD8+ T cell immunity in acute-resolving versus chronic viral hepatitis, in the next funding period, we will address these relevant questions to pursue the overall goal of identifying correlates of T cell stemness that can be exploited in therapeutic approaches e.g., by combining vaccination with stem-like T cells modulating agents. For this, we will
(i) in-vestigate the heterogeneity of stem-like virus-specific T cells applying longitudinal multi-modal single cell analysis (in collaboration with Wohlleber, Thimme, Neumann-Haefelin/Luxenburger and Z03)
(ii) determine intrinsic factors and circuits regulating stem-like potential applying computational and perturbation models (in collaboration with Bengsch, Knolle, Thimme and Z03)
(iii) pinpoint extrinsic factors preserving and modulating the stem-like virus-specific T cell pool applying in vitro stimulation assays and ex vivo spatial analysis (in collaboration with Böttler, Binder/Beisel, Knolle and Z02), during and after cure of chronic viral hepatitis in comparison to acute-resolving infections with hepatitis viruses (sample access via the FREEZE biobank HBUF hub).
Finally, we aim to define strategies targeting stem-like T cells in order to redirect the T cell response in chronic viral hepatitis (together with Protzer/Kosinska, Wohlleber, Knolle and Thimme).
Taken together, we are convinced that these results will increase our understanding of stemness in human T cell immunity and how it can be targeted, and thus translated, to steer the T cell response in immuno-therapeutic approaches such as HBV cure.